
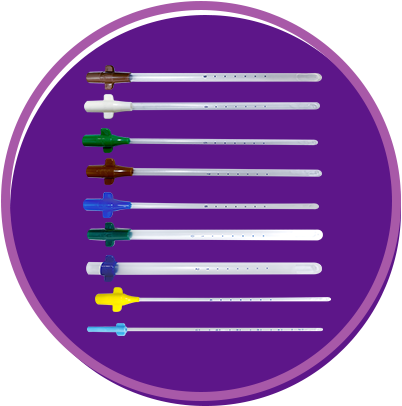
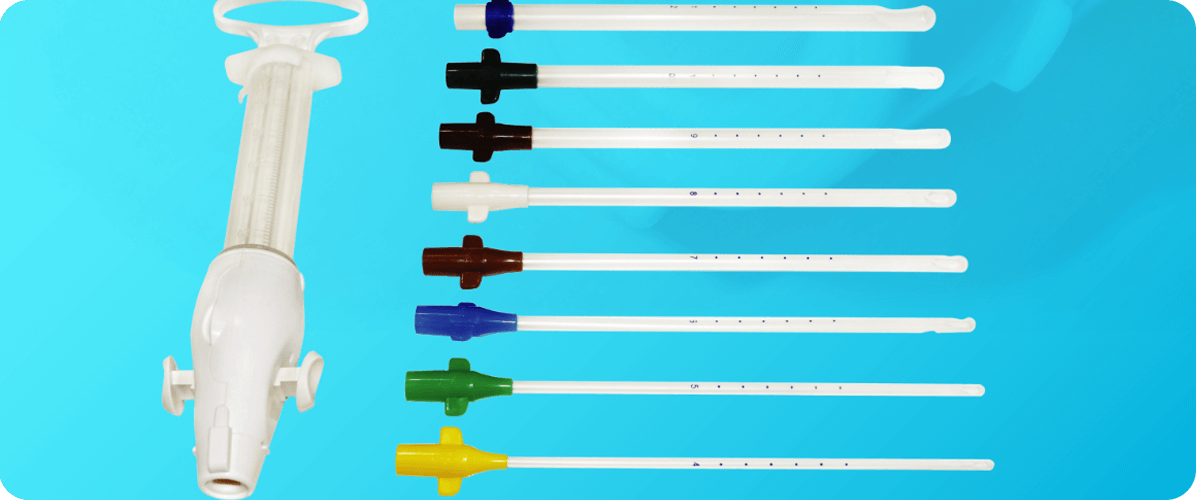
IPAS MVA is a safe simple and effective method for uterine evacuation. It is also well-suited for use at primary point of caredue to its low cost, simplicity and portability.
Clinical Application:
• Management of miscarriage
• Endometrial biopsy
• Treatment of Early Pregnancy Failure
• Evacuation of Hydatidiform Mole


A simple and effective method for uterine evacuation an endometrial biopsy
• Convenient Processing By
most standard methods, including steam autoclave and boiling.
• Ergonomic Design
Redesigned double-valve and plunger enhance ease of use.
• Enhanced Cleanability
Easy disassembly and reassembly Featuring a continual uid path.
• Highly Durable
Manufactured to high standards from top-quality materials.

The tactile response of a rigid curette with the gentle probe of a flexible Cannula
• Integrated Bases
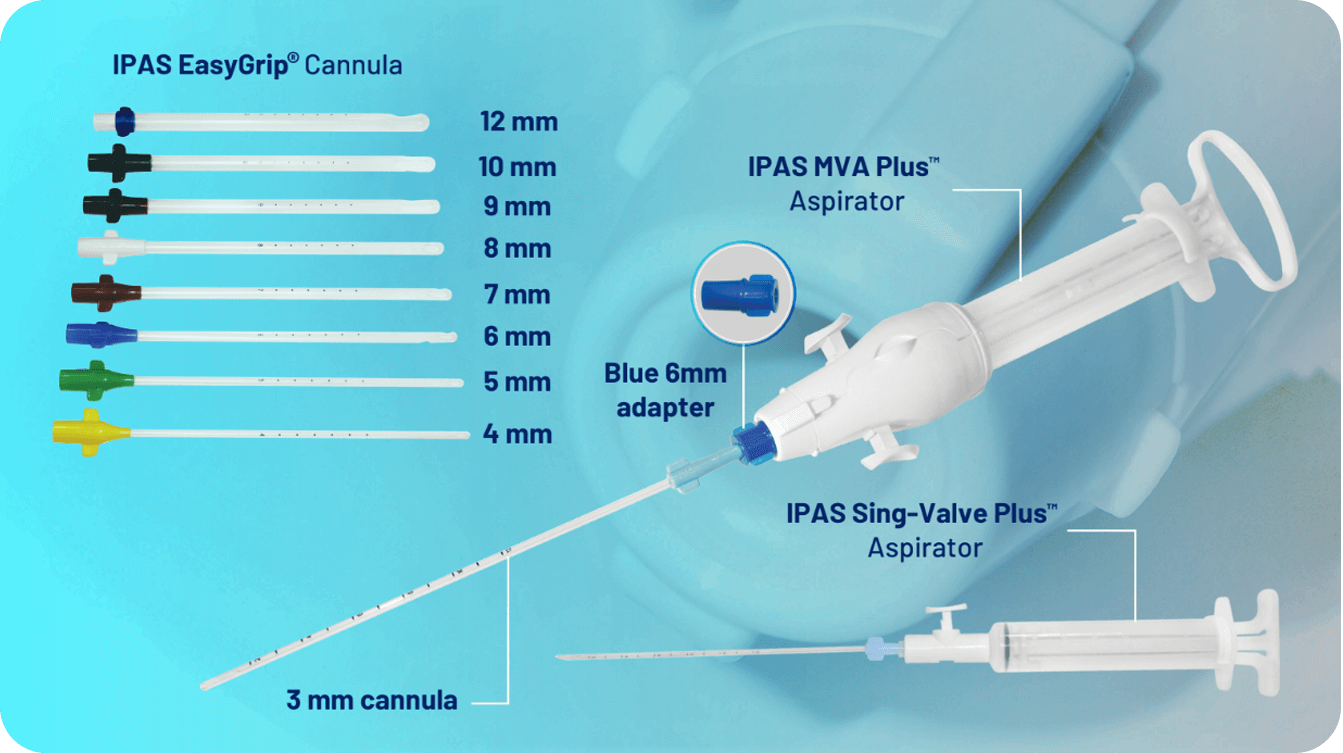
Eliminate the need for adapters Include wings for easy insertion and removal Color-coded by size.
• Technical Specications:
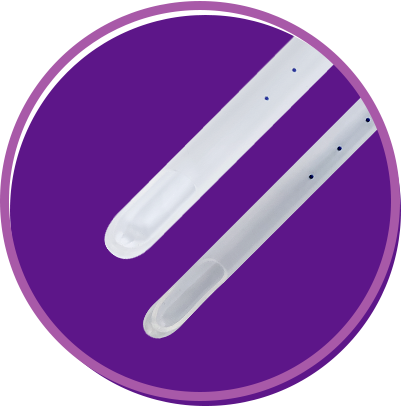
Length: 24 cm or 9 in Six marking dots starting at 6 cm from the tip and spaced 1 cm apart Time-tested apertures (one or two, depending on cannula size) Sizes: 4 mm to 12 mm
MVA is safe and a low-cost system.
• Less painful
• Less blood loss
• Less incidence of infection
• Less 60% cost on treatment of miscariage due to less time needed to complete the procedure, non-requirement for general anesthesia (GA) and shorter hospital stay.
MVA is safe and a low-cost system.
• Less painful
• Less blood loss
• Less incidence of infection
• Less 60% cost on treatment of miscariage due to less time needed to complete the procedure, non-requirement for general anesthesia (GA) and shorter hospital stay.


• Vacuum aspiration is the preferred method ofuterine evacuation for
miscarriages.
• Dilatation and sharp curettage, if still practiced, should be replaced by
vacuum aspiration.
• Strength of recommendation: strong
• “Evacuate the uterus with vacuum aspiration or medications, not sharp curettage.”

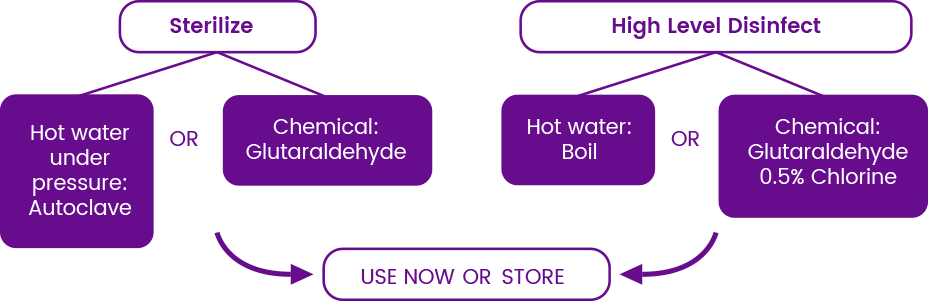
• Does not have to be sterile or HLD: does not contact the patient
• Aspirator can be used after cleaning
• Can be further processed after cleaning, if desiredength of recommendation: strong
• Cannulae must be HLD or sterilized
Immediately following the procedure, all Ipas MVA Plus Aspirators and Ipas EasyGrip Cannulae that will be reused should be kept wet until cleaning. Presoak, rinse or spray device with water or enzymatic spray. Do not use chlorine or saline.
Wear gloves and face protection. Disassemble and clean all instrument surfaces thoroughly in warm water and preferably detergent—not soap.